You run your business.
We will take care of the chemistry.
CONTINUOUS FLOW TECHNOLOGY
Chemical manufacturing made it simple.
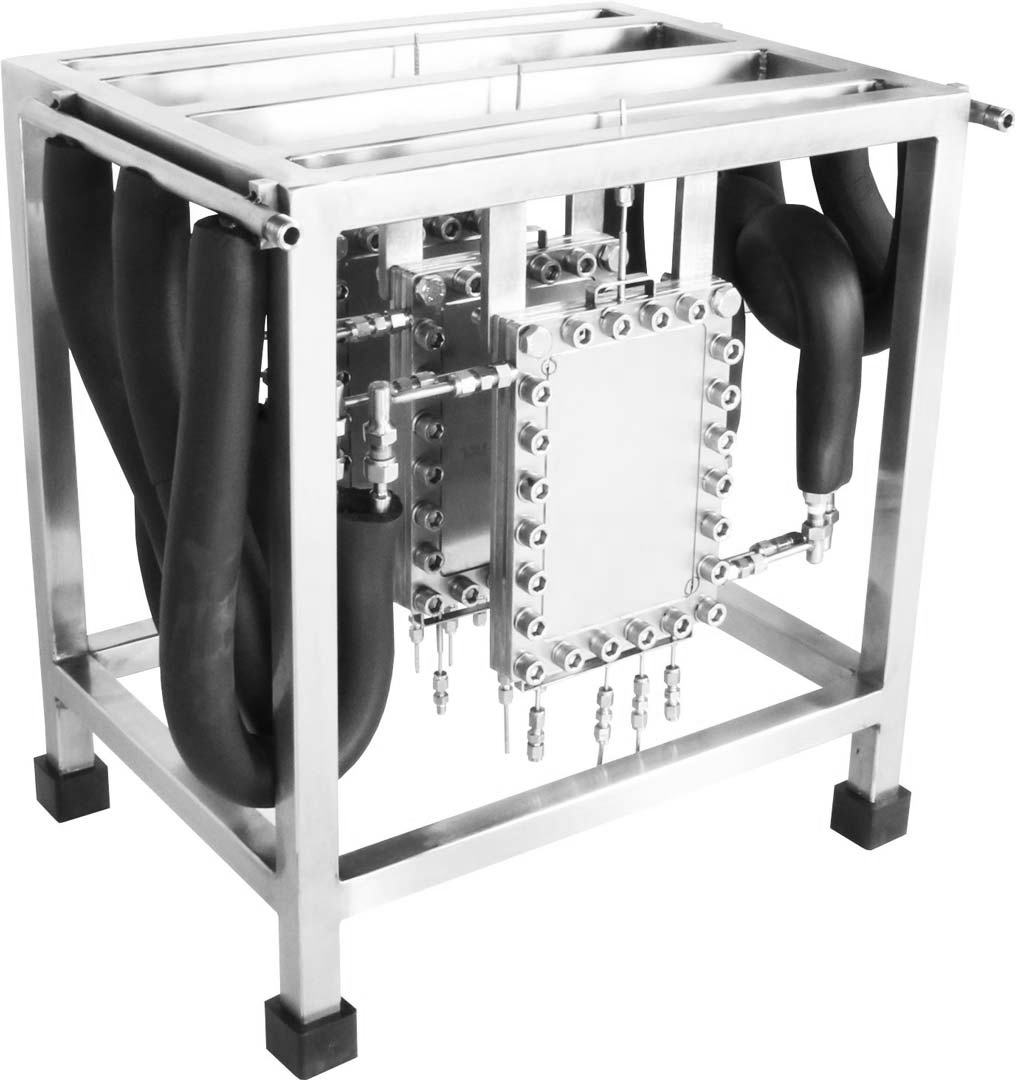
From the design of micro-reactors to container sized solutions, flow processing technology can shrink an entire chemical synthesis facility by the order of twenty-fold. This capacity, can be converted into huge advantages in CAPEX, enabling portable solutions for a very wide spectrum of chemistry applications.
With the capacity to deliver portable solutions, flow technology adds to Chemistry the just in time concept, for being able to synthesize products in fully automated plants anywhere in world.
This concept enables verticalization and modularization of the design of chemical plants, not only changing the way we do chemical synthesis today, but also setting a new standard for the supply chain of specialty chemicals for the next 100 years.
Our team has a very solid knowledge of chemical advanced technologies and we can guide your business towards the future of synthesis.
Smaller Reactors, Huge Advantages.
| - Huge CAPEX Advantages | - Faster & Safer Reactions |
| - Plug & Produce Concept | - Decentralized Production |
| - Modular Scalability | - Reduce Time to Market |
| - Full Mobility | - Taylor Made Processes |
| - Process Integration | - High Adaptable |
| - Fully Automated Process | - New Markets Readiness |
| - High Standardization | - Automated Production |
Ideas moves our business. We're fully opened to new projects.
Our specialists can work with your team finding the best solution.
Transparency and quality are Key values for us.
CDMO & PROCESS DEVELOPMENT
We design, scale-up and deliver your desired product.
With more than ten years of experience on chemical synthesis, our team of PhDs and researchers are fully capable to develop processes under batch or continuous-flow environment. We provide molecule development and manufacturing services in the industry on a contract basis (CMO Operations).
Our expertise covers from the lab-scale to industrial operations leading processes under very strict quality control procedures, as required to produce active pharmaceuticals ingredients (API's) according to good manufacturing practices (GMP), from the qualification of the raw material to production of the final Drug Master File under e-CDT rules, as required by FDA, Anvisa and other regulators worldwide.
10+ Years
of Experience in Process Development
CDMO
Contract & Development Manufacturing Capabilities
20+
Developed Process for Chemical Industry
Bring your idea
PhD researcher specialists on organic synthesis
Always focused on high quality products
QUALITY AND REGULATORY SOLUTIONS
Focus on quality and regulation integrated into process development
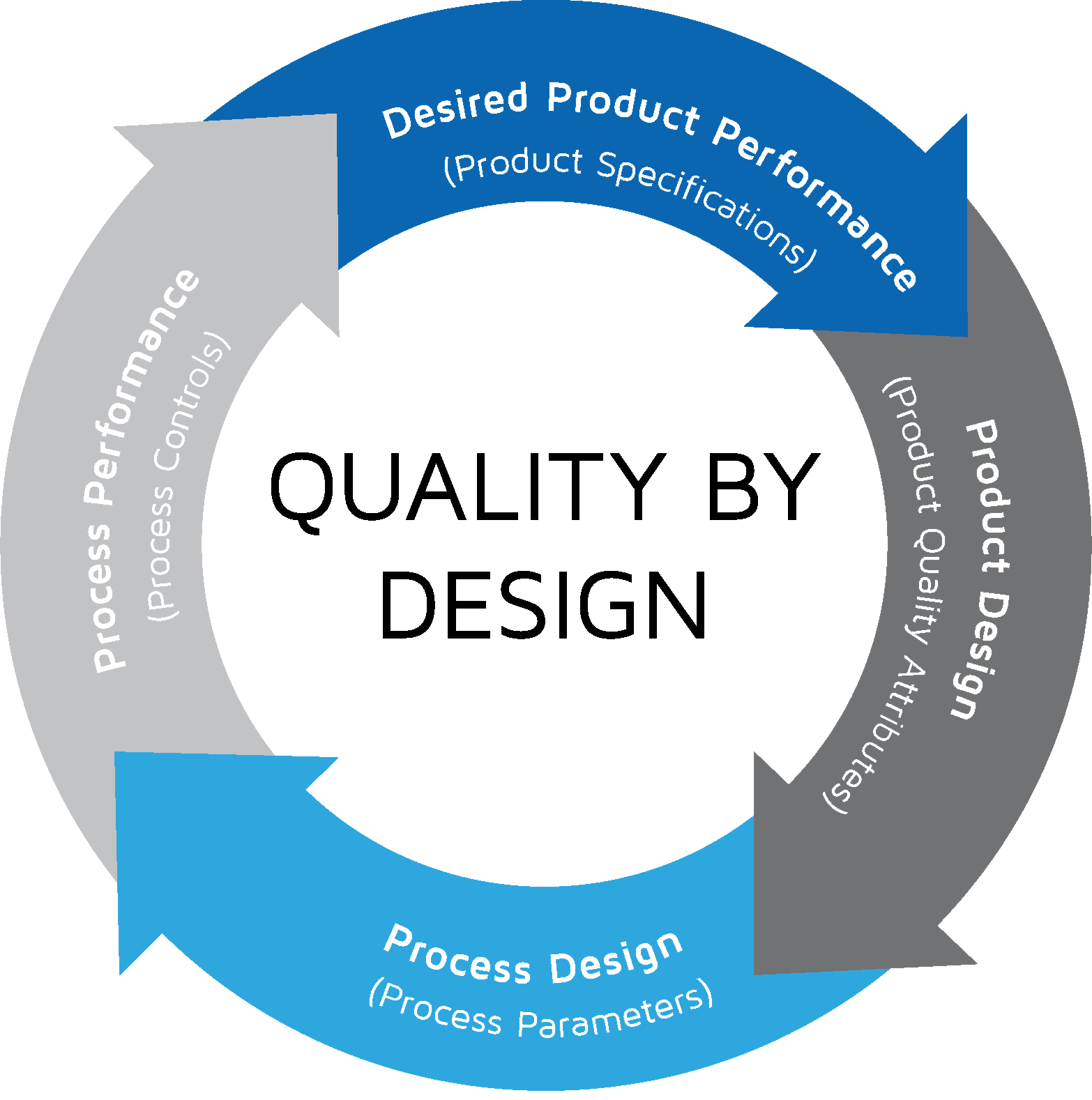
Designing with quality and innovation (QbD) is one of the three universal processes required to achieve breakthroughs in new products, services, and processes. Our quality department is integrated into the process development in order to qualify our process according to ICH-Q7 and ICH-Q13. Our team is also able to specify and qualify raw materials, intermediaries and products according to current regulations under demand. Quality and regulation is part of our DNA.
Quality by design approach itegrated with process development
Specification and regulatory issues solved by our team
Always focused on high quality products
MARKET INTELLIGENCE
Data are complex. We are here to help you untangle it all.
Our Internal BI solution tracks 5+ years of Brazilian Imports & Exports regarding date, prices, quantities, freight, inbound and outbound ports and airports, cities and countries. We can also monitor government purchases, contract owners, prices and payments. Our Database comprehends more than 320 millions single entries categorized by CAS (NCM in South America), with groups and subgroups supporting search by Keywords under SaaS technology.
We also monitors market tendencies, medicine registries on countries as the USA - Orange Book (FDA), Brazil - Anvisa and other regulators helping our clients to find business opportunities.
BI Solutions
Data from Brazil trade, imports & exports and government purchases & contracts.
Market Analysis
Skilled professionals and studies on the last tendency on markets.
See beyond the numbers, and find what is important for your business.
Hire a professional and use your time with data analysis, not software development.
Hire a professional and use your time with data analysis, not software development.